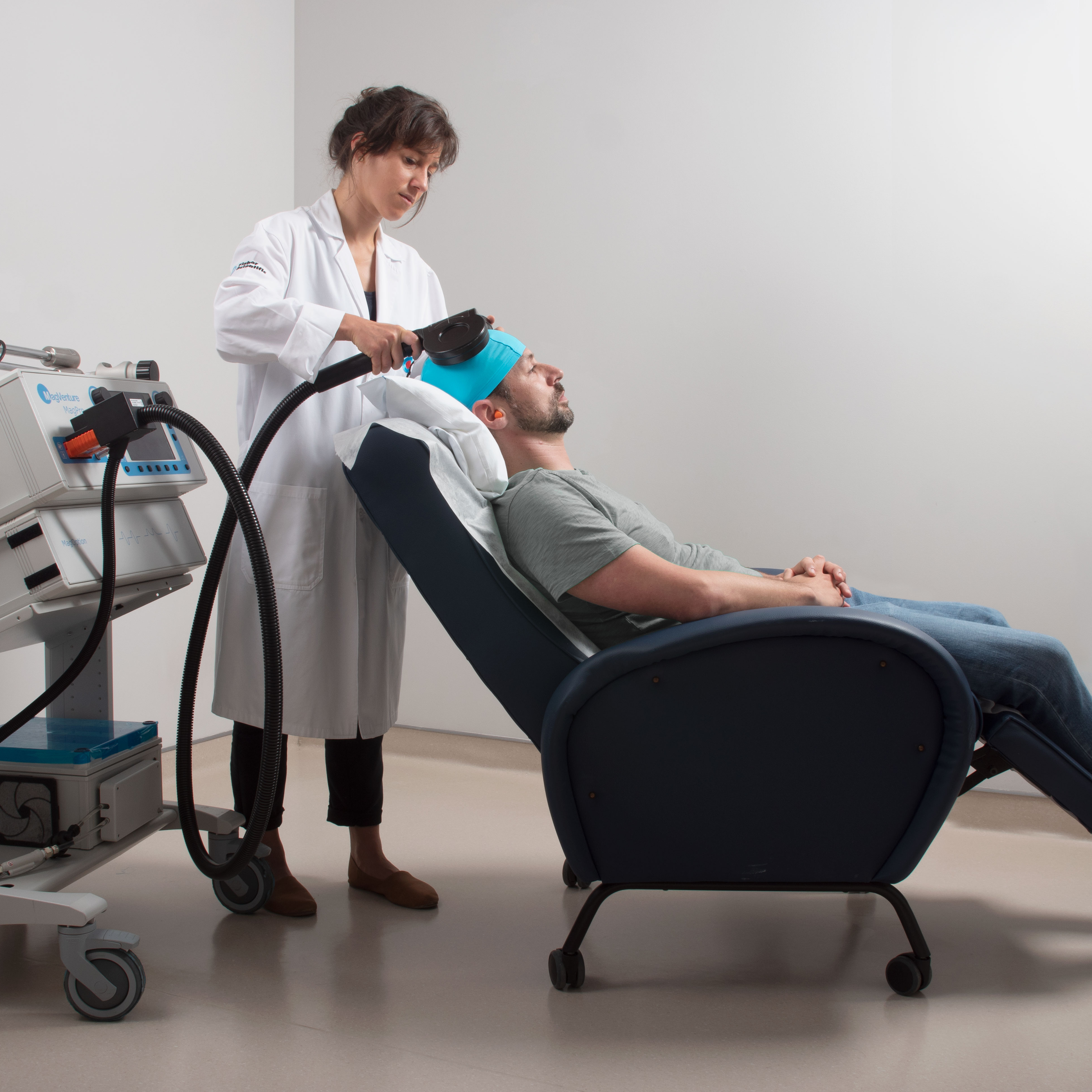
Since the 1990s, non-invasive brain stimulation has grown from an experimental idea into an established tool for treating depression as well as advancing neuroscience research. Among these technologies, repetitive transcranial magnetic stimulation (rTMS) is clinically validated and used to treat major depressive disorder, also known as unipolar depression. In the case of bipolar depression – a phase of bipolar disorder which is also characterized by phases when mood swings from bottom-less lows to extreme and risky highs – treatment options remain scarce and conventional antidepressants could trigger these dangerous manic episodes.
Motivated by a Breakthrough Device Designation for rTMS in bipolar depression (BDep), delivered by the U.S. Food and Drug Administration (FDA), clinicians and researchers from Champalimaud Foundation’s Neuropsychiatry Unit conducted a rigorous and comprehensive review of the available literature on rTMS for BDep – evaluating its efficacy, safety and optimal stimulation protocols. Published in Biological Psychiatry: Global Open Science, their findings offer renewed hope for patients and new guidance for clinicians. The clinical relevance of this work was also highlighted by a comment from a Canadian colleague in the same journal.
What Makes Bipolar Depression so Challenging to Treat
Depression can range from sadness to severe, debilitating episodes affecting multiple domains and symptoms. However, in patients with bipolar disorder, depressive episodes alternate with manic episodes - periods of extreme energy, impulsivity, risky behaviours and reduced need for sleep.
“During a severe manic episode, people may stop sleeping, or make very expensive and impulsive purchases, among other behaviours” explains Albino Oliveira-Maia, psychiatrist, neuroscientist and senior author of the review with Gonçalo Cotovio, also a psychiatrist, and who coordinates TMS activities at the Champalimaud.
Standard antidepressants, the cornerstone of depression treatment, can trigger mania in some bipolar patients. “The consequences can be devastating”, explains Gonçalo Cotovio. This severely limits therapeutic options, with clinicians relying on combinations of mood stabilisers, antipsychotics and psychotherapy. Despite these efforts, many patients continue to struggle with persistent, treatment-resistant depressive episodes.
Why rTMS is Changing The Treatment Landscape For BDep
With rTMS already approved as an effective and safe alternative for the treatment of unipolar depression, there was hope for its use to treat bipolar depression. However, more evidence was needed to prove rTMS's efficacy and safety for this challenging condition. In 2020, acting on the urgent need for better treatments, the FDA granted TMS, Breakthrough Device Designation for BDep, signaling their active support to accelerate research and regulatory approval. Around the same time, the Champalimaud Foundation team was starting to train psychiatrists and residents, both from Portugal and other countries, in TMS, expanding capacity for neuromodulation treatments.
“When we started, we were the only TMS clinic in Portugal", says Albino J. Oliveira-Maia. “Although we are very proud of that fact, it is something we felt compelled to change, training colleagues and publishing on standard methodology for clinical use of TMS across several disorders1,2,3”.
Two of the earliest TMS trainees at the Champalimaud, Fabiana Ventura and Pedro Frias, co-authors of the recently published review, have been involved in establishing TMS units within hospitals in the Portuguese National Health System. This growing network of trained clinicians set the stage for a rigorous, large-scale evaluation of TMS for bipolar depression.
Building on the FDA designation and years of pedagogic efforts, the authors of the review undertook compiling all available information to determine the best course of action.
The Review: What the Evidence Actually Shows about rTMS
From an initial pool of over 1000 scientific articles, researchers systematically analysed 56 studies involving more than 1500 patients with bipolar depression, comparing real rTMS with sham (placebo-like) stimulation. Analysis of the data in these articles revealed that:
TMS is effective for bipolar depression. Indeed, response and remission rates were similar to those described for unipolar depression and significantly better than sham treatment. Critically, TMS did not increase the risk of mania compared to sham, a crucial safety profile for patients with bipolar disorder, and side effects, such as mild, temporary headaches or fatigue were uncommon.
“The risk for treatment-emergent mania is a significant problem for conventional anti-depressant medication in bipolar depression”, referred Albino J. Oliveira-Maia. “It is quite re-assuring to see that this does not seem to be the case for treatment with TMS”.
TMS is a versatile approach, with multiple evidence-based protocols, including high-frequency and low-frequency stimulation of the dorsolateral prefrontal cortex (DLPFC), and intermittent theta burst stimulation (iTBS), which has the advantage of allowing for shorter treatment sessions. Effectiveness was compared between the several methods. “iTBS performed just as well as standard rTMS,” noted Gonçalo Cotovio, an important finding for clinics looking to optimize treatment in this population.
Finally, this review also revealed that patients with more severe baseline depression often had the most significant improvements, which has also been described for unipolar depression. However, contrary to findings in unipolar depression, TMS effectiveness did not seem to diminish in patients who had failed multiple prior therapies.
“This indicates real potential for difficult-to-treat cases.” Gonçalo Cotovio added.
A New Chapter: The Digital Neurotherapeutics Centre
While the review highlights strong evidence that rTMS is safe, effective and a versatile treatment option for bipolar depression, it also underscores the need for more head-to-head comparisons with existing treatments and for refinement of stimulation protocols, for BDep and other disorders.
The new Digital Neurotherapeutics (DNTx) Centre at the Champalimaud Foundation offers TMS, such as iTBS and accelerated protocols, to patients with depression, including bipolar depression, obsessive-compulsive disorder and chronic pain, among other conditions.
By integrating and bridging clinical care with research, the DNTx Centre will optimize neuromodulation treatments and expand access to safe, evidence-based brain-stimulation, for the patients that most need these treatments.
Text by Thaïs Lindemann, Neurotechnology Liaison Officer in Communication, Events and Outreach Team, and in the CRN Coordination Team at the Champalimaud Foundation.
__